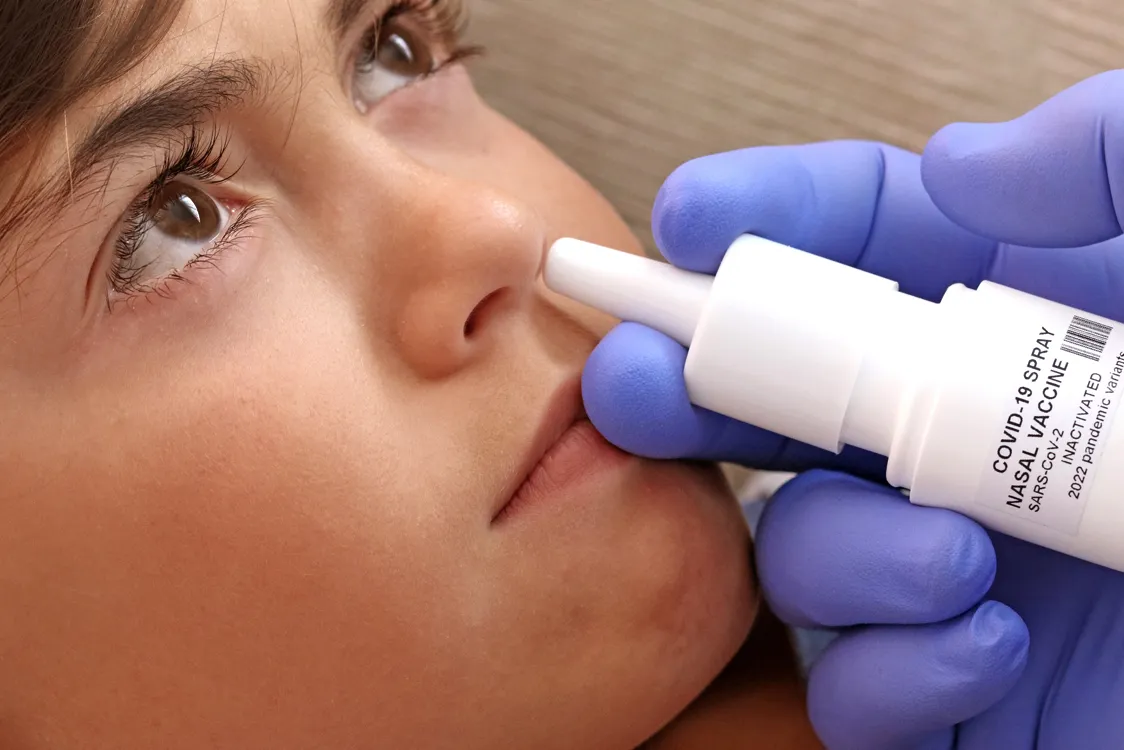
Bharat Biotech’s intranasal COVID vaccine gets emergency use authorization from the Drug Controller General of India (DGCI) on Tuesday, informed Union Health Minister Dr. Mansukh Mandaviya in a tweet.
Bharat Biotech’s ChAd36-SARS-CoV-S COVID-19 (Chimpanzee Adenovirus Vectored) recombinant nasal vaccine is approved by CDSCO for primary immunization against COVID-19 in the 18+ age group for restricted use in an emergency.
Informing that this will be India’s first nasal vaccine for COVID-19, Union Health Minister Dr. Mansukh Mandaviya called it a ‘Big Boost to India’s Fight AgainstCOVID-19’.
He further informed that the step would strengthen India’s collective fight against the pandemic.
“India has harnessed its science, R&D, and human resources in the fight against COVID-19 under PM @NarendraModi Ji’s leadership. With the science-driven approach & Sabka Prayas, we will defeat COVID-19,” he added.